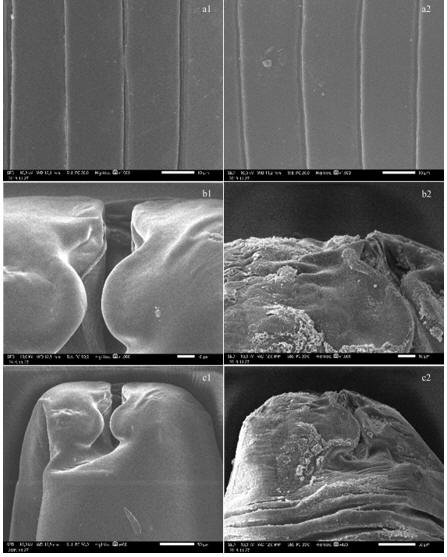
region of body, b1, c1: anterior end), destruction of the ultrastructure of Ascaridia galli soaked in CLE
at a concentration of 140 mg/mL (a2: cuticle in posterior region of body, b2, c2: anterior end)
| Livestock Research for Rural Development 33 (7) 2021 | LRRD Search | LRRD Misssion | Guide for preparation of papers | LRRD Newsletter | Citation of this paper |
Parasitic worms that live and develop in the gastrointestinal tract are often found in poultry. Ascaridia galli is the most common type of these worms. Commercial anthelmintic drugs cause many problems including environmental pollution, adverse effects on host health, and widespread anthelmintic resistance. As an alternative, clove leaves are very valuable as an anthelmintic because they contain eugenols, tannins, saponins, flavonoids. This study aims to determine the anthelmintic potential of the clove-leaf ethanol extract (CLE) against Ascaridia galli. A total of 240 worms were divided into 8 groups to be treated with CLE at seven different concentrations (20, 40, 60, 80, 100, 120, and 140 mg/mL) and saline as control, respectively with 3 repetitions. Ten worms of each group submerged into 25ml of the test solution for 3-9 hours, then evaluated using a Scanning Electron Microscope (SEM). The results showed the highest mean mortality at 3h, 6h, and 9h observation was CLE 140 mg/mL. The ethanol extract of clove leaves caused surface changes resulting in damages to the cuticle of A. galli adult worm. It can be concluded that CLE possesses anthelmintic activity.
Keywords: herbal, SEM, worm ultrastructure
Parasitic worms that live and develop in the gastrointestinal tract are often found in poultry. Ascaridia galli is the most common type of these worms (Fahrimal and Raflesia 2002). The prevalence of chickens infected with Ascaridia galli can reach 92% (Offionga et al 2013). Ascaridia galli can be found in broilers, layers, local chickens, and ducks (Jayentakumar Singh and Mohilal 2017). Many commercial anthelmintic drugs are used to control the incidence of ascaridiosis (Mubarokah et al 2019).
Commercial anthelmintic drugs cause many problems including environmental pollution, adverse effects on host health, and widespread anthelmintic resistance (Vercruysse et al 2011). The exploitation of medicinal plants provides a culturally acceptable alternative disease control option as well as ecological and environmentally friendly (Nalule et al 2011). Plants of the genus Syzygium from comparative studies revealed that they can have gastrointestinal activity against infectious agents, as anthelmintics (Patel et al 2010).
Clove (Syzygium aromaticum) is known as a native Indonesian plant that is used in kretek cigarette, food, beverage, and medicinal industries (Cortés-Rojas et al 2014; Jannah et al 2020). Nowadays it is also cultivated in India, Madagascar, Sri Lanka, the South of China, Tanzania, and other countries in the world (Bhuiyan et al 2010; Kamatou et al 2012). Generally, cloves are marketed in 2 main products: dried clove shoots and essential oil extracted from shoots, leaves or stems (Vernin et al 1994). In traditional Chinese medicine, clove oil has been used as a carminative, antispasmodic, antibacterial, and anthelmintic, while the buds are used to treat dyspepsia, gastritis, and diarrhea (Barceloux, 2008; Harborne and Baxter 1993). The most important active compound in clove essential oil is eugenol (Srivastava et al 2017).
Clove leaves are very valuable as an anthelmintic because they contain eugenols, tannins, saponins, flavonoids. Clove leaves contain about 1–4% essential oil. The composition of the resulting essential oil varies depending on the state of the clove leaves and the way of distillation, the resulting oil usually contains eugenol between 80 - 88%. Clove-leaf oil in Indonesia has a concentration of 75-77% eugenol (Razafimamonjison et al 2014). The phytochemical content of clove-leaf powder contains saponins, tannins, alkaloids, glycosides, and flavonoids (Nurdjannah 2004; Nurdjannah et al 1993).
Therefore, this study was conducted to determine the anthelmintic potential of clove-leaf ethanol extract (CLE) against Ascaridia galli through the proportion of phytochemical content of clove-leaf ethanol extract (CLE) and the effect of CLE the mortality rate and ultrastructure of adult Ascaridia galli.
This research was conducted in November 2019 and observed in Laboratory of Veterinary Internal Medicine, Faculty of Veterinary Medicine, Universitas Gadjah Mada. Clove leaves were obtained from Magelang Regency, Central Java Province.
Ethical approval was not applicable in this study as samples used for to collect Ascaridia galli were sourced from slaughtered chickens from poultry abattoirs.
Samples were weighed at ± 50 mg, extracted with 10 mL diethyl ether for 20 hours, and filtered. The remaining diethyl ether was then evaporated. Distilled water was added to the sample to reach the volume of 10 mL. The tests used 1 mL of sample, in which 0.1 mL of Folin-Ciocalteu reagent was added and vortexed for 5 minutes. The solution was added with 2 mL of 20% sodium carbonate, vortexed for 5 minutes, added with distilled water to a volume of 10 ml, diluted 5 times and read the absorbance at λ 760 nm after incubation for 30 minutes at room temperature (Chanwitheesuk et al 2004).
The sample was weighed at 50 mg, added with 1 ml of water, and extracted in a vortex for 5 minutes. The solution was added with 50 µl of anisaldehyde, shaken, incubated for 10 minutes at room temperature, then added with 2 ml of 50% sulfuric acid, heated in a water bath at 60°C for 10 minutes. The solution was then added with water to a volume of 10 ml, diluted 10 times, then the absorption was read at λ 435 nm.
The sample was weighed at 0.10 g and put into a 10 ml test tube containing 0.3 ml of 5% sodium nitrite. After 5 minutes, the sample was added with 0.6 ml of 10% aluminum chloride. After another 5-minute incubation, 2 ml of 1 M sodium hydroxide and distilled water were added to bring the volume to 10 ml. The solution was diluted 25x, then the absorption was read at λ 510 nm.
Samples were diluted with 0.5 ml of Folin-Ciocalteu reagent and 7.5 ml of bi-distilled water. The mixture was incubated for 10 minutes at room temperature to allow complete mixing, then added with 1.5 ml of 20% sodium carbonate and bi-distilled water to a volume of 10 ml. The solution was diluted as needed, then the absorption was read at λ 760 nm.
The clove leaves obtained were dried for 30 to 36 hours and then oven-dried for an additional 6 hours at a temperature of 45˚C. Samples that had been dried were ground to produce flour/powder measuring at 60 mesh. A total of ± 1 kg powder would be extracted by maceration/immersion technique. The solvent used was 96% ethanol. The ratio of the sample in the form of flour to ethanol was 1: 3. Soaking was done in the shaker for 2 hours, then incubated for an additional 12 hours. The extraction results were filtered, placed in a rotary evaporator to remove the ethanol, and oven-dried at 60° C for approximately 24 hours to obtain a dry extract.
To study the effect of clove-leaf extract (CLE) on Ascaridia galli , extracts (initially dissolved in DMSO) were diluted in distilled water at the desired concentrations for use in different assays. A total of 240 worms were divided into 8 groups, CLE was prepared at seven different concentrations (20, 40, 60, 80, 100, 120, and 140 mg/ml) and saline as control, respectively with 3 repetitions. Ten worms in each group were submerged into 25ml of the tested solution for 3-9 hours, then evaluated using a Scanning Electron Microscope (SEM).
The specimen preparation process consisted of 5 stages, starting from cleaning the worms by soaking in a cacodylate buffer for 2 hours followed by agitation in an Ultrasonic cleaner for 5 minutes. Afterwards, these samples went through prefixation, fixation, dehydration, and drying. The dried samples were placed in a test tube, coated with copper in an ion coating machine for 15 minutes and observed with a Scanning Electron Microscope.
Mortality data obtained were statistically analyzed using PROC FREQ in SAS version 9.0. and continued with Tukey test for any differences detected.
Table 1 shows the percentage of phytochemical contents of clove leaves extracted using ethanol.
|
Table 1. Phytochemicals of clove-leaf extract |
||
|
Parameters |
% w/w |
|
|
Tannin |
2.39 |
|
|
Saponin |
6.97 |
|
|
Flavonoid |
6.39 |
|
|
Phenol |
18.0 |
|
Based on table 1, it shows the percentage concentration of phytochemicals of clove leaves ethanol extract with a tannin content of 2.39%, 6.97% saponins, 6.39% flavonoids, and a total phenol of 18.0%.
|
Table 2. Mean percent mortality of Ascaridia galli after immersion for 3-9h |
|||||||
|
Treatments |
Mortality (%) |
SEM |
p value |
||||
|
3h |
6h |
9h |
|||||
|
Saline water |
0.00d |
0.00d |
0.00d |
6.70 |
0.0001 |
||
|
20 |
0.00d |
0.00d |
3.33d |
||||
|
40 |
0.00d |
0.00d |
10.0cd |
||||
|
60 |
0.00d |
6.67cd |
13.3cd |
||||
|
80 |
3.33d |
16.7cd |
40.0bc |
||||
|
100 |
3.33d |
30.0cd |
70.0ab |
||||
|
120 |
20.0cd |
33.3cd |
83.3a |
||||
|
140 |
40.0bc |
73.3ab |
93.3a |
||||
|
a,b,c,d Different superscripts in column and row indicate significant differences (p<0.05) |
|||||||
The treatment of various concentrations of CLE solution showed significant differences at concentrations of 80, 100, 120, 140 mg/mL (Table 2). Meanwhile, the time of death showed a significant difference at 3h, 6h, and 9h. CLE at the concentration of 20 mg/mL did not show a significant difference from the control. The highest percentage of worm mortality was in treatment with the concentration of 140 mg/mL after 9h immersion.
The active phytochemical components found in clove leaves comprise of alkaloids, tannins, saponins, flavonoids, phenols, glycosides, and terpenoids (Madubuike et al 2018). The amounts of these compounds are influenced by various factors, namely species, varieties, growing conditions, seasonal variations, processing, and storage methods (Pyo et al 2014). The present study carried out on the ethanol extract of clove leaves (Syzygium aromaticum) revealed that there is a presence of medicinal active constituents: 2.39% tannins, 6.97% saponins, 6.39% flavonoids, and 18.04% phenol. The phenol quantification is an important step in drug discovery (Madhavan and Tharakan 2020).
Meanwhile, the beneficial effects of medicinal plants usually result from secondary products present in plants, usually not attributed to only one compound but a combination of metabolites (Parekh et al 2005). However, clove oil is known to contain several compounds other than eugenol (up to about 85-90%) that have antifungal, anesthetic and antiseptic properties, such as eugenol acetate that makes up 9-10% (Madubuike et al 2018). Several studies have revealed that eugenol is an antiparasitic agent with potential effects on the growth, viability, and morphology of different parasites, such as Giardia lamblia, Leishmania donovani, and Trypanosoma cruzi (Machado et al 2011; Islamuddin et al 2014; De Morais et al 2014; Santoro et al 2007)
 |
| Figure 1.
The ultrastructure of Ascaridia galli not soaked in
clove-leaf extract (CLE) (a1: cuticle in posterior region of body, b1, c1: anterior end), destruction of the ultrastructure of Ascaridia galli soaked in CLE at a concentration of 140 mg/mL (a2: cuticle in posterior region of body, b2, c2: anterior end) |
As A. galli mortality rate is demonstrated in Table 2, Figure 1 shows the damage and wrinkling to the anterior side of the worm observed in SEM. This proves that there is anthelmintic activity of CLE ( Syzygium aromaticum) against Ascaridia galli. The anthelmintic activity of these plant extract is related to the presence of biologically active metabolites such as condensed tannins, flavonoids, steroids, terpenoids, alkaloids and saponins (Hoste et al 2006; Vargas-Magaña et al 2014). Tannins can attack A. galli either directly or indirectly. Tannin affects A. galli directly by attaching to the cuticle (Spiegler et al 2017). Tannin and two flavonoid compounds, namely quercetin and luteolin, work together to inhibit the growth of L3 H. concortus larvae in vitro (Zhong et al 2014). The antioxidants present in phenol have anthelmintic efficacy in stimulating worm death (Klongsiriwet et al 2015). For nematodes, saponins have been associated with the formation of complexes with cellular membrane components present at various stages of the nematode life cycle, leading to increased membrane permeability and causing these parasites to die (Srivastava et al 2017; Doligalska et al 2011). It was reported that the steroid saponin-rich and triterpenic fraction of Agave sisalana had an 'in vitro' ovicidal effect against goat nematodes (Vo et al 2017).
Clove-leaf ethanol extract (CLE) contains active compounds that can cause death and ultrastructural changes in Ascaridia galli. Therefore, clove leaves have the potential to be anthelmintic.
This study was supported by The Ministry of Education, Culture, Research, and Technology of the Republic of Indonesian through Pendidikan Magister Menuju Doktor Untuk Sarjana Unggul (PMDSU). The authors also thank to Universitas Gadjah Mada, Indonesia for the support.
Barceloux D G 2008 Medical Toxicology of Natural Substances. Foods, Fungi, Medicinal Herbs, Plants and Venomous Animals. Hoboken, Wiley.
Bhuiyan M N I, J Begum, N C Nandi and F Akter 2010 Constituents of the essential oil from leaves and buds of clove ( Syzygium caryophyllatum (L.) Alston). African Journal of Plant Science, 4, 451-454. https://doi.org/10.5897/AJPS.9000051
Chanwitheesuk A, A Teerawutgulrag and N Rakariyatham 2004 Screening of Antioxidant Activity and Antioxidant Compounds of Some Edible Plants of Thailand. Food Chemistry, 92, 491-497. https://doi.org/10.1016/j.foodchem.2004.07.035
Cortés-Rojas D F, C R Fernandes de Souza and W P Oliveira 2014 Clove (Syzygium aromaticum): a precious spice. Asian Pacific Journal of Tropical Biomedicine, 4, 90-96. https://doi.org/10.1016/S2221-1691(14)60215-X
De Morais S M, N S Vila-Nova, and C M Bevilaqua 2014 Thymol and euge-nol derivatives as potential antileishmanial agents. Bioorganic & Medicinal Chemistry, 22, 6250-6255. https://doi.org/10.1016/j.bmc.2014.08.020
Doligalska M, K Józwicka, M Kiersnowska, A Mroczek, C Paczkowski and W Janiszowska 2011 Triterpenoid saponins affect the function of P-glycoprotein and reduce the survival of the free-living stages of Heligmosomoides bakeri. Veterinary Parasitol, 179, 144-151.
Fahrimal Y and R Raflesia 2002 The degree of gastrointestinal nematode infestation in native chickens raised semi-intensively and traditionally, Journal of Medicine and Veterinary, 2, 114-118.
Harborne J B and H Baxter 1993 Phytochemical Dictionary. London, Taylor and Francis.
Hoste H, F Jackson, S Athanasiadou, S M Thamsborg and S O Hoskin 2006 The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends in Parasitology. 22, 253-261.
Islamuddin M, G Chouhan, M Tyagi, M Z Abdin, D Sahal and F Afrin 2014 Leishmanicidal activities of Artemisia annua leaf essential oil against visceral leishmaniasis. Frontiers in Microbiology, 5, 626. https://doi.org/10.3389/fmicb.2014.00626
Jannah M, J Muhidong and M Mursalim 2020 Physical Characteristics of Clove (Syzygium aromaticum). Jurnal Agritechno, 13, 34-41. https://doi.org/10.20956/at.v13i1.251
Jayentakumar Singh L and N Mohilal 2017 Gastrointestinal parasitic infection in diverse species of domestic birds of Manipur, India. Jounal of Parasitic Disease, 41, 142-146.
Kamatou G P, I Vermaak and A M Viljoen 2012 Eugenol from the remote Maluku Islands to the international market place: a review of a remarkable and versatile molecule. Molecules, 17, 6953-6981. https://doi.org/10.3390/molecules17066953
Klongsiriwet C, J Quijada, A R Williams, I Mueller-Harvey, E M Williamson and H Hoste 2015 Synergistic inhibition of Haemonchus contortus exsheathment by flavonoid monomers and condensed tannins. International Journal for Parasitology: Drugs and Drug Resistance, 5, 127-134. https://doi.org/10.1016/j.ijpddr.2015.06.001
Machado M, A M Dinis, L Salgueiro, J B Custódio, C Cavaleiro and M C Sousa 2011 Anti-Giardia activity of Syzygium aromaticum essential oil and eugenol: effects on growth, viability, adherence and ultrastructure. Experimental Parasitology, 127, 732-739. https://doi.org/10.1016/j.exppara.2011.01.011
Madhavan M and S T Tharakan 2020 Total Phenol Quantification and Anthelmintic Activity of Sarcostemma acidum (ROXB.) VOIGT. Journal of Pharmaceutical Science and Research, 12, 28-30.
Madubuike P, D Ezemokwe and N Madubuike 2018 Phytochemical Screening of Eugenia Caryophyllata (Clove Leaves) and Characterization of Its Essential Oil. World Wide Journal of Multidisciplinary Research and Development, 4, 163-167.
Mubarokah W W, W Nurcahyo, J Prastowo and K Kurniasih 2019 In vitro and in vivo Areca catechu crude aqueous extract as an anthelmintic against Ascaridia galli infection in chickens. Veterinary World, 12, 877-882. http://doi.org/10.14202/vetworld.2019.877-882
Nalule A S, J M Mbaria, D Olila and J W Kimenju 2011 Ethnopharmacological practices in management of livestock helminthes by pastoral communities in the drylands of Uganda. Livestock Research for Rural Development 23:6. http://www.lrrd.org/lrrd23/2/nalu23036.htm
Nurdjannah N 2004 Diversification of the Use of Cloves. Perspektif, 3, 61-70. (article in Bahasa)
Nurdjannah N, S Hardjo and Mirna Mirna 1993 Distillation method influence the yield and quality of clove leaf oil. Industrial Crops Research Journal, 3, 18-26.
Offionga E E A, O E Obiokub, J U Umohb, C A Essienc and N B Idiongc 2013 A survey of gastrointestinal helminths of local chickens in Abak local government area of Akwa Ibom State. International Journal of Sciences: Basic and Applied Research, 9, 1-4.
Parekh J, D Jadeja and S Chanda 2005 Efficacy of Aqueous and Methanol extracts of some medicinal plants for Potential Antibacterial Activity. Turkish Journal of Biology 29, 203-210.
Patel J, G S Kumar M S Qureshi, and P K Jena 2010 Anthelmintic activity of ethanolic extract of whole plant of Eupatorium adoratum. International Journal of Phytomedicine, 2, 127-132.
Pyo Y M, Y J Jin and J Y Hwan 2014 Comparison of the effect of blending and juicing on phytochemical content and antioxidant capacity of typical korean kernel fruit juice. Preventive Nutrition and Food Science, 19, 108-114. http://doi.org/10.3746/pnf.2014.19.2.108
Razafimamonjison G, M Jahiel, T Duclos, P Ramanoelina, F Fawbush and P Danthu 2014 Bud, leaf and stem essential oil composition of Syzygium aromaticum from Madagascar, Indonesia and Zanzibar. International Journal of Basic and Applied Sciences 3, 224-233.
Santoro G F, M G Cardoso, L G Guimarães, L Z Mendonça and M J Soares 2007 Trypanosoma cruzi: activity of essential oils from Achille amillefolium L, Syzygium aromaticum L and Ocimum basilicum L on epimastigotes and trypomastigotes. Experimental Parasitology. 116, 283-290. https://doi.org/10.1016/j.exppara.2007.01.018
Spiegler V, E Liebau and A Hensel 2017 Medicinal plant extracts and plant-derived polyphenols with anthelmintic activity against intestinal nematode. Natural Product Reports 34, 627-643.
Srivastava A, A K Gupta and A Rajendiran 2017 Phytochemical screening and in vitro anthelmintic activity of methanolic extract from the stem bark of Plumeria rubra linn.
International Journal of Pharmaceutical Sciences And Research, 5, 127-134. https://doi.org/10.13040/IJPSR.0975-8232.8(12).5336-41
Srivastava A K, S K Srivastava and K V Syamsundar 2005 Bud and leaf essential oil composition of Syzygium aromaticum from India and Madagascar. Flavour and Fragrance Journal, 20, 51-53. https://doi.org/10.1002/ffj.1364
Vargas-Magaña J J, J F J Torres-Acosta, A J Aguilar-Caballero, C A Sandoval-Castro, H Hoste and J I Chan-Pérez 2014 Anthelmintic activity of acetone-water extracts against Haemonchus contortus eggs: Interactions between tannins and other plant secondary compounds. Veterinary Parasitol, 206, 322-332.
Vercruysse J, M Albonico, J M Behnke, A C Kotze, R K Prichard, J S McCarthy, A Montresor and B Levecke 2011 Is anthelmintic resistance a concern for the control of human soil-transmitted helminths. International Journal of Parasitol : Drugs Drug Resist, 1, 14-27. https://doi.org/10.1016/j.ijpddr.2011.09.002
Vernin G, E Vernin, J Metzger, L Pujol and C Parkanyi 1994 GC/MS analysis of clove essential oils. In Spices Herbs and Edible Fungi. Amsterdam, Elsevier Science.
Vo N N Q, E O Fukushima and T Muranaka 2017 Structure and hemolytic activity relationships of triterpenoid saponins and sapogenins. Journal of Natural Medicine, 71, 50-58. https://doi.org/10.1007/s11418-016-1026-9
Zhong R Z, H X Sun, H W Liu and D W Zhou 2014 Effects of tannin acid on Haemonchus contortus larvae viability and immune responses of sheep white blood cells in vitro. Parasite Immunol. 36, 100-106.